radiation primer







|
Radiation is a hard-working word in physics. It describes several diverse natural processes and their effects. As used in common speech, it means what physicists call "ionizing radiation", or that which can produce detrimental effects in materials and organic tissue. Ionization is the process of removing electrons from atoms, and when this occurs in biological tissues it disrupts the delicate chemical and physical processes that sustain life. This can happen through mutation, when the DNA of the organism is altered, or directly via the destruction of atomic bonds and the breakup of important molecules at the site of the ionization.
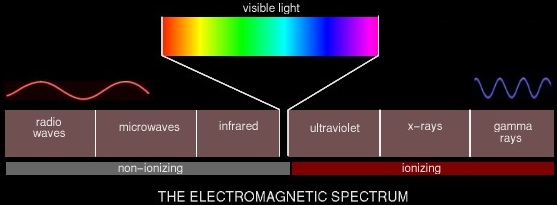
We consider two broad categories of ionizing radiation: that caused by electromagnetic rays, and that caused by high-energy charged particles.
As you can see, not all electromagnetic (EM) waves are ionizing radiation. Generally anything above the visible spectrum is considered ionizing radiation and thus harmful to some degree. Ultraviolet radiation from the sun is what sometimes causes skin cancer. X-rays and gamma rays are produced by nuclear reactions -- atomic bombs, and to a much lesser degree, nuclear reactors. Non-ionizing EM radiation can still be dangerous, of course, in sufficient quantities. Microwaves cook food by exciting the water molecules in the food until they vibrate and create heat. Obviously they can also excite the water molecules in the human body and cause a similar effect. The other category of ionizing radiation comprises high-energy charged particles.
A beta particle is an electron emitted from the nucleus of a radioactive substance. It has a charge of -1 and is much, much less massive than a proton or a neutron. A proton is, well, a proton. And neutrons are neutrons. Protons and neutrons have about the same mass, but the neutron doesn't have a charge while the proton has a charge of +1. Now having made a careful distinction between waves and particles, we note that many authors use the terms interchangeably (e.g., beta ray and beta particle). Since EM radiation is carried by the photon (a particle) and since equivalent energies can be computed for proper particles, there isn't any real need to maintain such a strict distinction. In fact, it's frequently useful to be able to share measurements between all the different kinds of radiation. But when computing radiation dosage (the effect of radiation on organisms) and when constructing shielding, the differences must be clearly understood. Pound for pound, particle radiation is much more dangerous than wave radiation. The bigger the particle, the more damage it is capable of doing.
We mentioned above that physicists deal with radiation in a more abstract concept. In their terminology, it is one of the mechanisms by which energy is transferred from one place to another. Radiation is "energy in transit". When we speak of the "energy" of a wave we consider its intensity. The energy of a particle can be thought of as equivalent to its speed. High-energy particles travel very fast, while low-energy particles travel slowly. Physicists use another measurement, "flux", to describe a sort of particle density. If many particles pass by a certain point in a given length of time, we say the flux is high. If few particles pass, the flux is low. "Flux" is the Latin word for "flow". If we take a cubic meter of space anywhere in the universe, we'll discover that it contains many particles of varying flux and energy. In general, flux and energy vary inversely. That means the higher the energy, the lower the flux. So if we look at the low-energy particles, we may find an enormous flux.
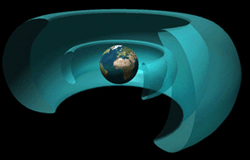
We can answer this question in two ways. We can say that charged particles come from the nuclei of various atoms that undergo nuclear decay. We can say that EM rays (especially x-rays and gamma rays) are emitted from those same nuclei, and we can note that any substance with sufficient energy, or heat, emits EM radiation as a method of releasing that energy. That describes the source of radiation at the microscopice level. But the pressing issue is where in the universe we might expect to encounter these types of radiation, and in what quantities. The short answer is that radiation is all around us. EM radiation bombards us constantly, but thankfully not generally in the ionizing range of wavelengths. High-energy charged particles rain down on us from space, and are produced by the natural radioactive decay of many natural substances. The constant low level radiation which we encounter every day is "background radiation". Predictably, the chief source of all kinds of radiation in space is the sun. A full spectrum of EM waves radiates outward from it. Charged particles of all types emanate from it, especially during periods of extreme solar activity (e.g., flares). Earth's atmosphere protects us from most ionizing electromagnetic radiation from the sun. Ultraviolet, x-ray, and gamma rays penetrate to some extent (enough to give us sunburns, for example), but in space there is a consistently higher level of all of these. But only during periods of extreme solar activity does this radiation exceed our ability to shield against it.
But when the sun acts up, the area outside the Van Allen belts becomes thick (i.e., high flux) with dangerous, high-energy charged particles. A solar event was depicted in the motion picture Red Planet, forcing the crew of that mission to seek cover. But since the Van Allen belts themselves contain concentrations of charged particles, going through them presents its own hazard. We can think of it as crossing a barbed-wire fence: the fence offers protection, but can also snag us as we crawl through it. We've left neutrons out of the picture up until now, and that's
because they just don't occur as high-energy particles anywhere
in the universe in numbers great enough to care about. Scientists
even have trouble creating them in the lab.
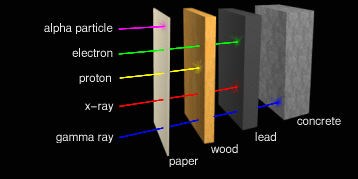
This is where the difference between radiation types becomes
important. Wave radiation requires thick, heavy shielding. It
requires considerably less material to block particles.
In general, the shorter the EM wavelength, the thicker and denser the
shield material must be. Ultraviolet (UV) can be blocked simply by a
sufficiently opaque sheet of plastic. We are all familiar with tinted
sunglasses that promise to block some 97% of solar UV rays. Not much
additional protection is required in space. X-rays and gamma rays are
another matter. Where intense x-rays and gamma rays occur, it
requires several inches or centimeters of lead and/or concrete to
provide adequate shielding.
Protons penetrate farther. They can be shielded by light metals or plastics in thicknesses of about a centimeter. Beta particles are very small and can penetrate centimeters into the body. But luckily they're too small to cause much damage if they hit anything. But there's a special problem here. When beta particles hit large atoms, the impact causes those atoms to give off x-rays. Metal atoms are usually quite heavy, and so are especially susceptible to this kind of re-radiation which is known by its German name "Bremsstrahlung". In fact, this is how x-rays are produced intentionally for medical applications. The best materials to shield against beta particles have lots of hydrogen atoms in them. Hydrogen atoms are light, and so absorb the particles without giving off x-rays. Plain old water works very well. In fact, 4 inches (10 centimeters) of water will block almost all background beta particles. But water is impractical for shielding in space, so high-density polyethylene (HPDE, chemical formula CH2CH2...) is frequently used instead. This also effectively blocks protons.
Radiation exposure is cumulative, meaning that the longer you're exposed to it, the worse effect it has. It's very much like running through the rain. We've discussed shielding, which is like an umbrella. But if it's impractical to provide complete shielding, you can also reduce the exposure time. This is the same as running through the rain rather than walking. If you forget your umbrella on a rainy day and have to park some distance from your destination, you can reduce your "exposure" to the rain by running from the car to the door. If you walk instead, you'll spend more time under the rain and thus get wetter. Organisms can recover from exposure to radiation, just as you can eventually dry after walking or running through the rain. The wetter you get, the longer it takes to dry. The more exposure to radiation, the harder it is to recover. The body will repair damage done to DNA or to other important molecules, although it will be sick in the meantime. It's actually better to absorb a high dose of radiation quickly than a low dose over a long period. Although the higher dose may cause more problems in the short term, the low dose will produce continuing damage and your body simply may not be able to keep up even though the damage is slight at any one moment. When it comes to designing space ships, additional shielding means additional weight, and that means your space ship may have to go slower. The answer to this tradeoff is to skimp on shielding and go faster. The radiation exposure will be more intense, but it will not last as long. This is preferred.
Most people aren't familiar with the various units and concepts used to measure radiation. It just isn't something they have to deal with. And so when conspiracists describe radiation using big numbers they find in textbooks and elsewhere, the general public isn't always equipped to understand what those numbers mean. The problem is exacerbated by the fact that Americans have one system of units for measuring things, and the rest of the world has another system. This is also true for radiation. So not only do people have to deal with labels they've never seen before, but they don't know what measurements are simply differences in units, a sort of radiological furlong versus a radiological centimeter.
Radiation activity is measured in an American unit called a "Curie" (Ci) or an international (SI) unit called a "Becquerel". The Curie is defined by how much radiation one gram of a radium isotope emits. The Becquerel just counts how many particles or photons (in the case of wave radiation) are emitted per second. The device used for measurement is often the familiar Geiger counter. If you put a Geiger counter over a gram of substance and count 3 clicks per second, the radioactivity of that substance would be 3 Bq. Radiation exposure is measured in American units by the "rad", an acronym standing for "radiation absorbed dose", and in the SI system by the Gray (Gy). The exposure is the amount of energy "deposited" in a substance by radiation. A rad is the amount of radiation required to deposit 100 ergs of energy in a gram of material. An erg is a very small amount of energy, but it takes only a very small amount of energy to ionize an atom. The number isn't important. The important concept is that exposure is measured by what radiation does to substances, not anything particular about the radiation itself. This allows us to unify the measurement of different types of radiation (i.e., particles and wave) by measuring what they do to materials. But what materials? Wood, water, human tissue -- they all have different densities, so a gram of one material may be bigger or smaller than a gram of another material. And the bigger something is, the more surface area is available for bombardment by rays or particles. It would be nice if there were some way of comparing exposure in various substances directly. Enter the "rem". That's another acronym, meaning "radiation equivalent, man". As with all measurements of exposure it describes the effects of radiation on substances that absorb it, but in this case the substance is specifically human tissue. It's an American unit; the corresponding SI unit is the Sievert (Sv). The reader is likely to encounter the term "Roentgen", which is another American unit of exposure. It measures the amount of ionization a certain amount of radiation produces in air, and has been largely abandoned in favor of the rad. It can be roughly equated to a rad for estimation purposes. Above we discussed that different kinds of radiation are inherently more dangerous than others. By measuring exposure in how it affects surfaces, we can largely ignore the differences in kinds of radiation. But in order to compute rems from rads we need to take into account that some kinds of radiation are inherently more dangerous to biological tissue, even if their "energy deposition" levels are the same. Each kind of radiation carries a "relative biological effectiveness" (RBE) factor, also called a "quality factor" (Q). For x-rays and gamma rays and electrons absorbed by human tissue, Q is 1. For alpha particles it is 20. For protons and neutrons, it is 10. To compute rems from rads, or Sieverts from Grays, simply multiply by Q. This is obviously a simplification. The RBE/Q factor approximates what otherwise would be very complicated computations. And so the values for Q change periodically as new research refines the approximations. Exposure occurs over time, of course. The more rems absorbed in a
unit of time, the more intense the exposure. And so we express actual
exposure as an amount over a specific time period, such as 100 rads
per hour, or 5 millisieverts per year. This is called the "dosage
rate", and is proportional to the flux of radiation in a particular
situation.
Conspiracy theorists exploit the natural radiophobia that has
arisen since the bombing of Japan with nuclear weapons, testing by
various nations, and the Chernobyl accident in the former Soviet
Union. But now that we understand a little bit about how radiation is
measured, we can quantify the danger.
The U.S. government endorses the recommendations of various
international regulatory bodies on the acceptable levels of radiation
exposure in the workplace and among the general public.
If a worker must deal with radioactive materials in the course of
his job, his legal limit is higher: 5 rem (50 millisieverts, mSv) per
year. If a worker is in the vicinity of radioactive materials but
does not work with them, the limit is 0.1 rem (1 mSv). For persons
younger than 18 and pregnant women, the occupational exposure is 0.5
(5 mSv) per year. These are measurements above the natural background
radiation limits, and are measured by dosimeters and other equipment
in the area where the exposure takes place. (Standards for
Protection Against Radiation. 10 CFR § 20.)
People usually get about 0.24 rem (2.4 mSv) in background
radiation per year. (Jawororwski, Zbigniew. "Radiation Risks in the
20th Century: Reality, Illusions, and Risks" Presented 17 Sept. 1998
at the International Curie Conference, Warsaw, Poland.)
The limits imposed by U.S. Federal Regulations are thus extremely conservative. The lethal dose is 700 times the amount of radiation acceptable per year for people who work around radioactivity. The regulations are so very strict because while it has been determined that even dosages up to 30 rems per year produce no visible effect, there is no such thing as radiation with no harmful effects. It just happens that for low doses the body can repair itself effectively. | ||||||||||||||||||||||||||||||||||||||