

THE MICROWATER UNIT
Tap water: What it is and isn't
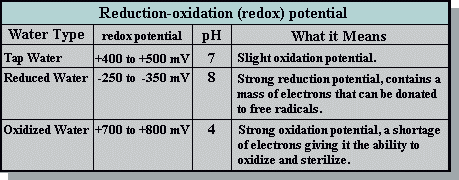
Normal tap water, for example, with a pH of 7 is approximately neutral on the pH scale of 0 to 14. When measured with an ORP (oxidation potential) meter its redox potential is approximately +400 to +500 mV. Because it has a positive redox potential, it is apt to acquire electrons and oxidize other molecules. Reduced microwater, on the other hand, has a negative redox potential of approximately -250 to -350 mV. This means it has a large mass of electrons ready to donate to electron-thieving active oxygen.
Before discussing the properties of microwater further, let's take a look at what happens inside the Microwater unit.
How the Microwater Unit works
The Microwater unit, slighty taller and thicker than a large dictionary on end, is an electrical appliance connected to your ketchen water supply to perform electrolysis on tap water before you drink it or use it in the kitchen for cooking or cleaning.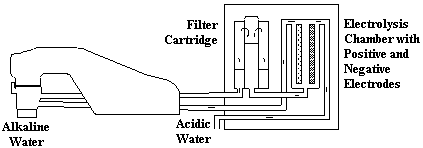
A special attachment re-directs tap water out of the faucet through a plastic hose into the Microwater unit. Inside the Microwater unit, the water is first filtered through activated charcoal. Next, the filtered water passes into an electrolysis chamber equipped with an platinum-coated titanium electrode where electrolysis takes place.
Cations, positive ions, gather at the negative electrodes to create cathodic water (reduced water). Anions, negatively charged ions, gather at the positive electrode to make anodic water (oxidized water).
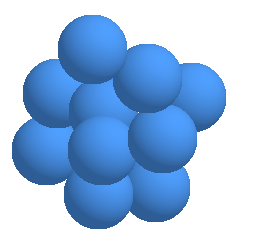
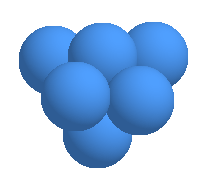
Through electrolysis, reduced water not only gains an excess amount of electrons
(e-), but the cluster of H2O seem to be reduced in size from about 10 to 13 molecules
per cluster to 5 to 6 molecules per cluster. ( see the graph on page 4. )
The reduced water comes out of the faucet, and the oxidized water comes out of a separate hose leading into the sink. You can use the reduced water for drinking or cooking. The oxidation potential of the oxidized water makes it a good sterilizing agent, ideal for washing hands, cleaning food or kitchen utensils, and treating minor wounds.
What the Microwater Unit Produces
Redox potential comparison
After electrolysis of the water inside the Microwater unit, reduced water comes out of the cathodic side and oxidized water comes out of the anodic side. Compare these measurements of these three types of water: tap water before electrolysis, the reduced water, and the oxidized water.
Redox potential, not pH, is the crucial factor
Traditionally we have judged the properties of water from the standpoint of pH, in other words whether water is acidic or alkaline. According to Dr. Yoshiaki Matsuo PhD., the inventor of the Microwater unit, "In my opinion, redox potential is more important than pH. The importance of pH is over emphasized. For example, the average pH of blood is 7.4 and acidosis or alkalosis are defined according to deviation within the range of 7.4 +- 0.005. But nothing has been discussed about ORP, or oxidation-reduction potential."
The pH of tap water is about pH 7, or neutral. When tap water is electrolyzed into microwater, its reduced water has a pH of about 9 and the oxidized water a pH of about 4. Even if you make alkaline water of pH 9 by adding sodium hydroxide or make acidic water of pH 3 by adding hydrogen chloride, you will find very little change in the ORP values of the two waters. On the other hand, when you divide tap water with electrolysis you can see the ORP fluctuate by as much as +- 1,000 mV. By electrolysis we can obtain reduced water with negative potential that is good for the body.

Proceed to page 4.
Microwater Menu page
NOTE:
Dr. Hayashi is a Heart Specialist and Director of the Water Institute of Japan.
Dr. Hayashi has no affiliation with "Total Health Marketing",
or "Microwater Systems".